WHY JUVÉDERM® IT?
- JUVÉDERM® is the world’s leading brand of hyaluronic acid (HA) facial fillers12,*
- Results with JUVÉDERM® can last between 9 and 24 months depending on the treatment chosen.13,14 JUVÉDERM® blends with the tissue underneath the skin, to give natural-looking results.15,17
* The no.1 hyaluronic acid brand according to market share in the majority of markets where data is available (2018).12
DISCOVER IT
The JUVÉDERM® range of injectable facial fillers designed to treat different areas of the face, depending on the results you would like to achieve.1-11
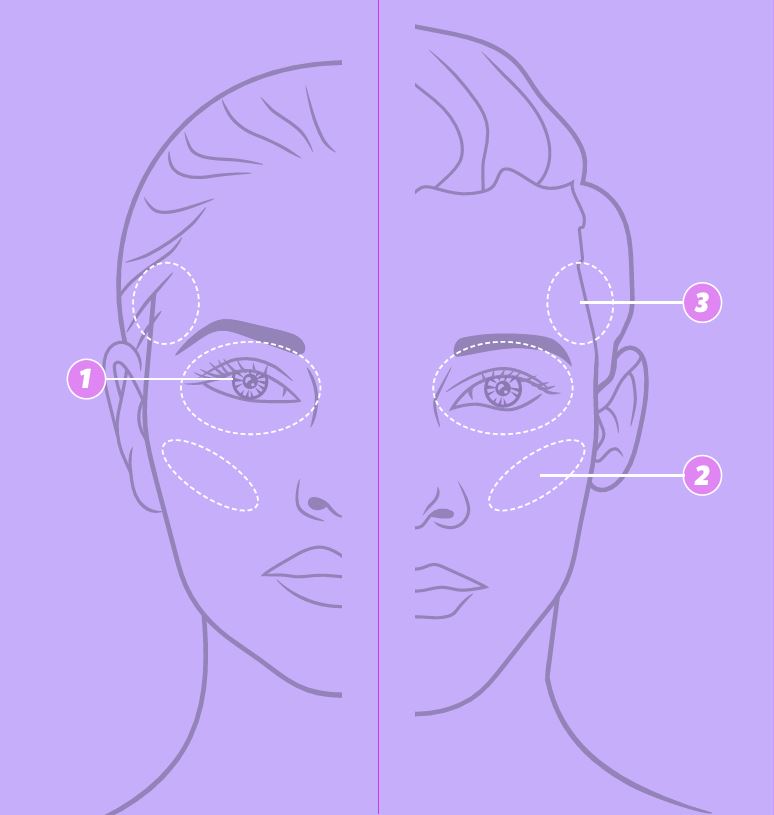
YOUR EYES. YOUR LOOK. ENHANCE YOUR EYES’ UNIQUE BEAUTY 1,2,4,*
Three JUVÉDERM® hyaluronic acid gels can be used in combination for a holistic treatment approach to the eye area.
Enhance the eye area and restore facial balance by not only treating the immediate periorbital area but your cheeks and temples too.30,32-36**
Create a customised treatment plan with your practitioner to achieve periorbital revitalisation and natural results.15,††

REFRESH THE UNDER-EYE AREA2,39
Distracting tear troughs can negatively influence the eye area look.31,33,34*
64% of patients (N=80) treated with Juvéderm® VOLBELLA† for the infraorbital area were satisfied up to 12 months post-treatment.39,¶

SMOOTH THE APPEARANCE OF YOUR CHEEKS1,40
Saggy cheeks can negatively influence the eye area look.29,34-41*
98% of patients (N=52) felt that their radial cheek lines were improved or much improved at Day 45 after treatment with Juvéderm® VOLIFT† vs baseline.40,‡,§

ENHANCE YOUR CHEEKS’ NATURAL CONTOURS4,14,42
Volume loss in the cheeks and temples can negatively influence the eye area look.34-36*
96% of patients (N=115) were satisfied with results from immediately after mid-face treatment with Juvéderm® VOLUMA.21,†
† Juvéderm® VOLBELLA, Juvéderm® VOLIFT and Juvéderm® VOLUMA contain lidocaine,1,2,4 the addition of which does not alter the physical properties of the products.29
†† In an in vivo study, a filler that has integrated with the tissue may give a natural look and feel.15
¶ As measured by the Global Aesthetic Improvement Scale (GAIS), patients who rated their eyes as ‘improved’, ‘well improved’ or ‘very well improved’.39
* Patients with cosmetic concerns regarding the periorbital area may first require indirect treatment of the cheeks and temples to address saggy cheeks and/or volume loss.34,35,38
‡ Primary effectiveness endpoint was the overall aesthetic improvement from Day 1 to Day 45 in radial cheek lines. Improvement was measured using the Global Aesthetic Improvement Scale (GAIS).40
§ The study met its primary effectiveness endpoint.40
HYDRATE IT3,13
Juvéderm® VOLITE
Our Skin‐Quality injectable treatment may improve hydration for up to 9 months.13,19 It can be used for hands, face, neck and décolletage to :20
- Improve elasticity3,19
- Improve the appearance of fine lines3,19
- Improve smoothness and even out skin depressions19
WHICH IS IT?
LIP DREAMS ARE MADE OF THIS…
Get the ideal Lip Look with JUVÉDERM® hyaluronic acid lip fillers.22-24 Experience lip results with a natural look and feel, lasting up to 12 months.15-17,25-28,*,†
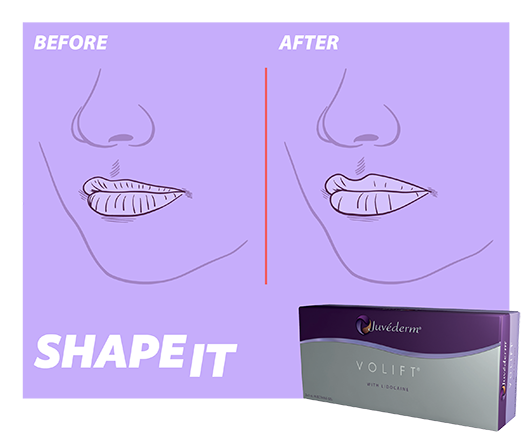
SHAPE IT with
Juvéderm® VOLIFT1,‡
For those seeking volumisation and lip natural shape enhancement1,22-24,28
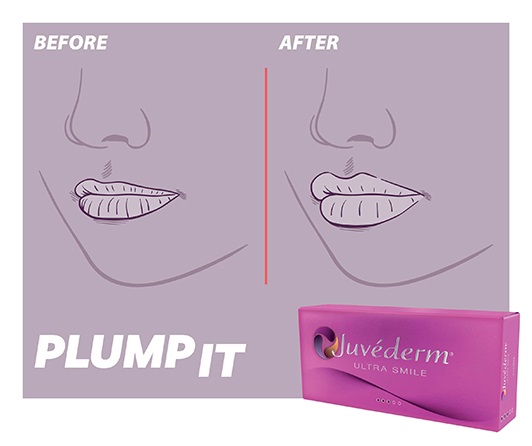
PLUMP IT with
Juvéderm® ULTRA SMILE6,‡
For those seeking a more visible change with extra lip volume6,23,24,27
SMOOTH IT with
Juvéderm® VOLBELLA2,‡
For those seeking subtle changes with improved lip definition and smoothness2,16,22-24
Study was conducted without lidocaine.16
† After treatment, Juvéderm® VOLIFT with lidocaine improved lip fullness for up to 12 months in 39% of patients (N=36), including those who received top-up treatment.28 In the study, 60 patients received initial treatment overall; patients could receive touch-up treatment at Day 14.28
‡ Juvéderm® VOLIFT with lidocaine and Juvéderm® VOLBELLA with lidocaine will be referred to as Juvéderm® VOLIFT and Juvéderm® VOLBELLA, respectively.
Juvéderm® VOLIFT, Juvéderm® ULTRA SMILE and Juvéderm® VOLBELLA contain lidocaine,1,2,6 the addition of which does not alter the physical properties of JUVÉDERM® products.29
GET IT RIGHT
A personal treatment plan will be created, based on your individual goals and you will be advised on the right quantity of JUVÉDERM® treatments needed to achieve results.
SEE IT RIGHT AWAY
ARRANGE IT
Create a treatment plan based on your personal goals, together with your local practitioner. Find out more about the consultation process or locate your nearest clinic here. Please be advised that there is a potential for side effects with JUVÉDERM® fillers.1-11 Consult your healthcare provider with any concerns.
Reference
1. Juvéderm® VOLIFT with lidocaine. 73652JT10. Revision 2019-09-09 (v0.1) 2. Juvéderm® VOLBELLA with lidocaine DFU. 73363JS10. Revision 2020-07-10. 3. Juvéderm® VOLITE with lidocaine. 73655JT10. Revision 2019-09-09 (v0.1) 4. Juvéderm® VOLUMA with lidocaine. 73650JT10. Revision 2019-09-09 (v0.1) 5. Juvéderm® VOLUX. 73651JT10. Revision 2019-09-09 (v0.1) 6. Juvéderm® ULTRA SMILE. 73664JT10. Revision 2019-09-09 (v0.1) 7. Juvéderm® ULTRA 2. 73661JT10. Revision 2019-09-09 (v0.1) 8. Juvéderm® ULTRA 3. 73662JT10. Revision 2019-09-09 (v0.1) 9. Juvéderm® ULTRA 4. 73663JT10. Revision 2019-09-09 (v0.1) 10. Juvéderm® ULTRA XC DFU. 11. Juvéderm® ULTRA PLUS XC DFU. 12. Allergan. DOF INT/0771/2016(2). Juvéderm is the world’s leading HA brand. Feb 2019. 13. Allergan. Data on File. INT/0297/2017. Juvéderm® VOLITE Clinical Study (V12-001) – 9 months topline summary. Apr 2017. 14. Jones D and Murphy DK. Dermatol Surg. 2013; 39:1602–1612. 15. Hee CK et al. Dermatol Surg. 2015; 41:373–381. 16. Eccleston D and Murphy DK. Clin Cosmet Investig Dermatol. 2012; 5:167–172. 17. Philipp-Dormston WG et al. J Cosmet Dermatol. 2014; 13:125–134. 18. Juvéderm® VOLIFT RETOUCH with lidocaine. 73653JT10. Revision 2019-09-09 (v0.1) 19. Niforos F et al. Poster Presentation at Beauty Through Science (BTS) Congress, Stockholm, Sweden; 2017. 20. Allergan. Unpublished Data. INT/0078/2017(1). Juvéderm® VOLITE treatment area. Mar 2019. 21. Philipp‐Dormston WG et al. J Cosmet Laser Ther. 2014; 16:171–179. 22. Greene RM. Facial Plast Surg. 2019;35:134–39. 23. Scanlon C. Journal of Aesthetic Nursing. 2015;4 Suppl. 5:24–30. 24. Miller A. Journal of Aesthetic Nursing. 2018;7 Suppl. 2:12–20. 25. Allergan. Unpublished Data. INT-JUV-1950095. Juvéderm® tissue integration images. Sep 2019. 26. Baumann LS et al. Dermatol Surg. 2007;33(Suppl 2):S128–35. 27. Lanigan S. J Cosmet Dermatol. 2011;10:11–14. 28. Allergan Aesthetics. Unpublished Data. INT-VOF-2050002. Juvéderm® VOLIFT for lip augmentation. 12-month data. Nov 2020 29. Raspaldo H et al. J Cosmet Dermatol. 2010;9:11–15. 30. Mcalees M. Journal of Aesthetic Nursing. 2020;9:154–55. 31. Brackenbury J. Journal of Aesthetic Nursing. 2019;8:436–40. 32. Swift A et al. Aesthet Surg J. 2021:1–13. doi: 10.1093/asj/sjaa339. Epub ahead of print. 33. Ziai K et al. Plast Aesthet Res. 2020;7:53: 1–13. 34. Haswell N et al. Journal of Aesthetic Nursing. 2021: 10;106–10. 35. Lipko-Godlewska S et al. Clin Cosmet Investig Dermatol. 2021;14:169–78. 36. Raspaldo H. Dermatol Surg. 2012;38:261–65. 37. Wieczorek IT et al. J Drugs Dermatol. 2015;14:1043–51. 38. Montes JR et al. Facial Plast Surg Clin North Am. 2021;29:335–48. 39. Niforos F et al. Dermatol Surg. 2017;43:1271–80. 40. Ogilvie P et al. Dermatol Surg. 2020;46:376–85. 41. Finn JC and Cox S. Facial Plast Surg Clin North Am. 2007;15:123–32. 42. Callan P et al. Clin Cosmet Investig Dermatol. 2013;6:81–89.